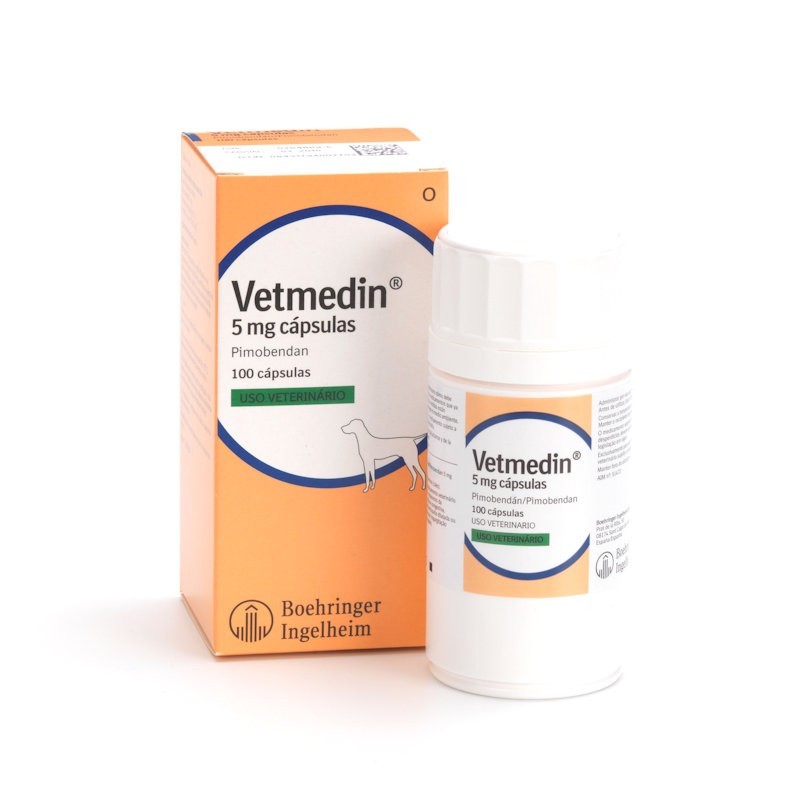
Vetmedin 5 mg hard capsules Species: Dogs Therapeutic indication: Pharmaceuticals: Cardiovascular and respiratory preparations Active ingredient: Pimobendan Product:Vetmedin® 5 mg hard capsules Product index: Vetmedin hard capsules Presentation Hard capsules white to orange in colour, containing 5 mg pimobendan per capsule as active substance and titanium Dioxide (E171) 1.2320 mg/capsule and Sunset Yellow (E110) 0.3080 mg/capsule as excipients. Uses For the treatment of canine congestive heart failure originating from valvular insufficiency (mitral and/or tricuspid regurgitation) or dilated cardiomyopathy. When used in cases of valvular insufficiency in conjunction with frusemide, the product has been shown to improve the quality of life and extend life expectancy in treated dogs. When used in a limited number of cases of dilated cardiomyopathy in conjunction with frusemide, enalapril and digoxin, the product has been shown to improve the quality of life and to extend life expectancy in treated dogs. Dosage and administration See dosing table below. Vetmedin 5 mg Capsules Dosage Guide No. of 5 mg capsules per administration Bodyweight (kg) Daily Dosage (mg) Morning Evening 21-40 10 1 1 41-60 20 2 2 >60 30 3 3 Vetmedin capsules should be administered orally (approximately one hour before feeding) at a dose of 0.2 mg to 0.6 mg pimobendan/kg body weight per day. The daily dose should be divided into two equal administrations, one half of the dose given in the morning and the other half approximately 12 hours later. Determine the bodyweight accurately before prescribing to ensure administration of the correct dosage. In cases of mild congestive heart failure, a daily dosage at the lower end of the dose range may be adequate. If, however, a clear response is not observable within one week, the dosage should be raised. Vetmedin capsules may be combined with a diuretic treatment such as frusemide. Contra-indications, warnings, etc Vetmedin capsules should not be used in cases of hypertrophic cardiomyopathies or clinical conditions where an augmentation of cardiac output is not possible for functional or anatomical reasons (e.g. aortic stenosis). Special precautions for use in animals The product should only be used in dogs with cardiac insufficiency. Do not exceed the recommend dosage. Special precautions to be taken by the person administering the veterinary medicinal product to animals In case of accidental ingestion, seek medical advice immediately and show the package leaflet or label to the physician. A moderate positive chronotropic effect and vomiting may occur in rare cases. However, these effects are dose-dependent and may be avoided by reducing the dose in these cases. In rare cases transient diarrhoea, anorexia or lethargy have been observed. In studies with rats and rabbits, pimobendan had no effect on fertility and embryotoxic effects occurred only at maternotoxic doses. In experiments with rats it has been shown that pimobendan is excreted into milk. No information is available on the safety of Vetmedin in pregnant and lactating bitches. Therefore, Vetmedin capsules should only be administered to pregnant and lactating bitches if the expected therapeutic benefits outweigh the potential risk. The pimobendan-induced increase in contractility of the heart is attenuated in the presence of the calcium antagonist verapamil and the β-antagonist propranolol. In pharmacological studies no interaction between the cardiac glycoside ouabain and pimobendan was detected. In the case of overdose, symptomatic treatment should be initiated. Pharmaceutical precautions Shelf life of the veterinary medicinal product as packaged for sale : 3 years. Do not store above 25°C. Store in a dry place. Store in tightly closed original container. Keep out of the reach and sight of children. For animal treatment only. To be supplied only on veterinary prescription. Any unused veterinary medicinal product or waste materials derived from such veterinary medicinal products should be disposed of in accordance with local requirements. Legal category Legal category: POM-V