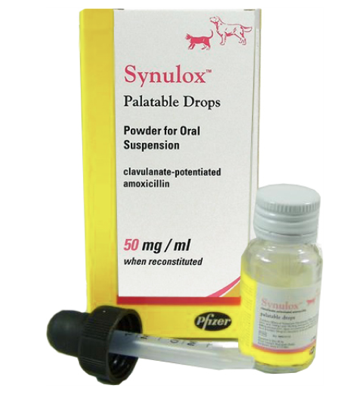
Synulox Palatable Drops, Powder for Oral Suspension Species: Cats, Dogs Therapeutic indication: Pharmaceuticals: Antimicrobials: Oral preparations: Others Active ingredient: Amoxicillin Trihydrate, Clavulanic Acid Product:Synulox® Palatable Drops Product index: Synulox Palatable Drops Presentation Synulox Palatable Drops are presented in a bottle as an off-white powder, containing 150 mg potassium clavulanate equivalent to clavulanic acid and 600 mg Amoxicillin trihydrate equivalent to amoxicillin. Reconstitution of the powder with 15 ml of water provides a suspension containing 10 mg clavulanic acid and 40 mg amoxicillin per ml. Uses Synulox Palatable Drops have a notably broad spectrum of bactericidal activity against bacteria commonly found in dogs and cats. In vitro Synulox is active against a wide range of clinically important aerobic and anaerobic bacteria including: Gram-positive: Staphylococci (including β-lactamase producing strains); Clostridia; Corynebacteria; Peptostreptococcus spp; Streptococci Gram-negative: Bacteroides spp. (including β-lactamase producing strains); Escherichia coli (including most β-lactamase producing strains); Salmonellae (including β-lactamase producing strains); Bordetella bronchiseptica; Campylobacter spp; Fusobacterium necrophorum; Klebsiellae; Pasteurellae; Proteus spp. Clinically Synulox has been shown to be effective in treating a wide range of diseases of cats and dogs including: Skin disease (including deep and superficial pyodermas); soft tissue infections (abscesses and anal sacculitis); dental infections (eg gingivitis); urinary tract infections; respiratory disease (involving upper and lower respiratory tract); enteritis. Dosage and administration Administration By the oral route. Reconstitution Add 15 ml water. Shake the bottle before use. Dosage rate 12.5 mg/kg bodyweight (i.e. 0.25 ml/kg). For the accurate dosing of particularly small patients it is valuable to note that one drop from the pipette provided contains 2.3 mg clavulanate-potentiated amoxicillin. Therefore 5–6 drops/kg twice daily are recommended as a guide. Dosage frequency Dogs and cats should be dosed at the rate of 0.25 ml of reconstituted product per kg bodyweight twice daily. For the majority of infections, including those of the skin, urinary tract and gastrointestinal tract, the above dosing regime is effective. Refractory cases, however, particularly those of the respiratory tract, have shown improved cure rates by doubling the dose to 25 mg/kg bodyweight twice daily (i.e. 0.5 ml of reconstituted product per kg bodyweight twice daily). Duration of therapy Routine cases involving all indications The majority of these cases respond to between 5 and 7 days therapy. Chronic or refractory cases In these cases, where there is considerable tissue damage, a longer course of therapy may be required in that it allows sufficient time for damaged tissue to repair. Based on clinical trials, the following durations are suggested as guidelines: Chronic skin disease 10–20 days Chronic cystitis 10–28 days Respiratory disease 8–10 days Contra-indications, warnings, etc Synulox Palatable Drops should not be given to rabbits, guinea pigs, hamsters or gerbils. Caution is advised in their use in any other very small herbivores. In very rare cases the use of the product may result in occasional instances of gastro-intestinal disorders (vomiting, diarrhoea, anorexia). In very rare cases hypersensitivity (allergy, allergic skin reactions) may occur after use. Allergic reactions may occasionally be serious (anaphylaxis). If allergic reactions occur, the product should be discontinued immediately. Appropriate symptomatic treatment should be initiated. The frequency of adverse reactions is defined using the following convention: - very common (more than 1 in 10 animals treated displaying adverse reaction(s)) - common (more than 1 but less than 10 animals in 100 animals treated) - uncommon (more than 1 but less than 10 animals in 1,000 animals treated) - rare (more than 1 but less than 10 animals in 10,000 animals treated) - very rare (less than 1 animal in 10,000 animals treated, including isolated reports). Operator warning Penicillins and cephalosporins may cause hypersensitivity (allergy) following injection, inhalation, ingestion or skin contact. Hypersensitivity to penicillins may lead to cross-reactions to cephalosporins and vice-versa. Allergic reaction to these substances may occasionally be serious. Do not handle this product if you know you are sensitised, or if you have been advised not to work with such preparations. Handle this product with great care to avoid exposure, taking all recommended precautions. If you develop symptoms following exposure, such as skin rash, you should seek medical advice and show the doctor this warning. Swelling of the face, lips or eyes or difficulty with breathing are more serious symptoms and may require urgent medical attention. Wash hands after use. Pharmaceutical precautions Powder: Do not store above 25°C. Reconstituted suspension: Store at 2-8°C. Any reconstituted suspension remaining 7 days after preparation should be discarded. Dispose of any unused product and empty containers in accordance with guidance from your local waste regulation authority. Keep out of the sight and reach of children. For animal treatment only. Legal category Legal category: POM-V