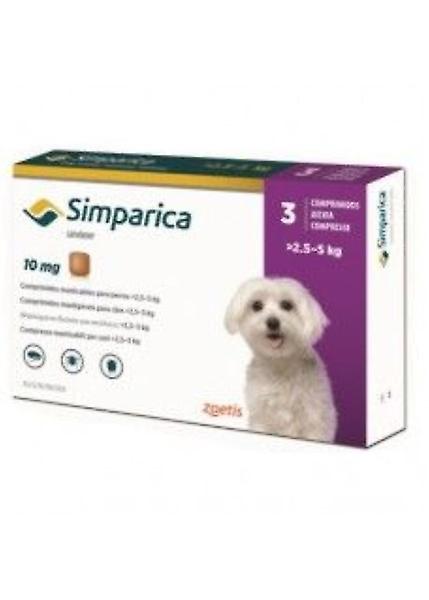
Simparica chewable tablets for dogs Species: Dogs Therapeutic indication: Pharmaceuticals: Ectoparasiticides: For dogs Active ingredient: Sarolaner Product:Simparica® chewable tablets for dogs Product index: Simparica chewable tablets for dogs Presentation Simparica chewable tablets are brown, square tablets with 5, 10, 20, 40, 80 or 120 embossed on one side. Each tablet contains: Simparica chewable tablets sarolaner (mg) for dogs 1.3 - 2.5 kg 5 for dogs >2.5 - 5 kg 10 for dogs >5 - 10 kg 20 for dogs >10 - 20 kg 40 for dogs >20 - 40 kg 80 for dogs >40 - 60 kg 120 Uses For the treatment of flea infestations (Ctenocephalides felis and Ctenocephalides canis) in dogs. Simparica has immediate and persistent flea killing activity against new infestations for at least 5 weeks. Simparica can be used as part of a treatment strategy for the control of Flea Allergy Dermatitis (FAD). For the treatment of tick infestations (Dermacentor reticulatus, Ixodes hexagonus, Ixodes ricinus and Rhipicephalus sanguineus). Simparica has immediate and persistent tick killing activity for at least 5 weeks. For the treatment of sarcoptic mange (Sarcoptes scabiei). For the treatment of ear mite infestations (Otodectes cynotis). For the treatment of demodicosis (Demodex canis). Fleas and ticks must attach to the host and commence feeding in order to be exposed to the active substance. Dosage and administration For oral use. Tablets can be administered with or without food. Simparica should be administered at a dose of 2-4 mg/kg bodyweight in accordance with the following table: Bodyweight (kg) Tablet strength (mg sarolaner) Number of tablets to be administered 1.3 - 2.5 5 One >2.5 - 5 10 One >5 - 10 20 One >10 - 20 40 One >20 - 40 80 One >40 - 60 120 One >60 Appropriate combination of tablets Use appropriate combination of available strengths to achieve the recommended dose of 2–4 mg/kg. Simparica tablets are chewable and palatable and readily consumed by dogs when offered by the owner. If the tablet is not taken up voluntarily by the dog it can also be given with food or directly into the mouth. The tablets should not be divided. Treatment schedule: For optimal control of flea and tick infestations, Simparica should be administered at monthly intervals and continued throughout the flea and/or tick season based on local epidemiological situations. For the treatment of ear mite infestations (Otodectes cynotis) a single dose should be administered. A further veterinary examination 30 days after treatment is recommended as some animals may require a second treatment. For the treatment of sarcoptic mange (caused by Sarcoptes scabiei var. canis) a single dose should be administered at monthly intervals for two consecutive months. For the treatment of demodicosis (caused by Demodex canis) the administration of a single dose once a month for three consecutive months is efficacious and leads to a marked improvement of clinical signs. Treatment should be continued until skin scrapings are negative on at least two consecutive occasions one month apart. As demodicosis is a multi-factorial disease, it is advisable to also treat any underlying disease appropriately. Contra-indications, warnings, etc Do not use in cases of hypersensitivity to the active substance or to any of the excipients. Parasites need to start feeding on the host to become exposed to sarolaner; therefore, the transmission of infectious parasite-borne diseases cannot be excluded. In the absence of available data, treatment of puppies less than 8 weeks of age and/or dogs less than 1.3 kg bodyweight should be based on a benefit-risk assessment by the responsible veterinarian. Mild and transient gastrointestinal signs such as vomiting and diarrhoea, and systemic disorders such as lethargy, anorexia/inappetence may occur in very rare cases (less than 1 animal in 10,000 animals treated, including isolated reports) based on post-marketing safety experience. These signs typically resolve without treatment. Neurological disorders such as tremor, ataxia or convulsion may occur in very rare cases based on post-marketing safety experience. In most cases these signs are transient. The safety of Simparica has not been established during pregnancy and lactation or in animals intended for breeding. Laboratory studies in rats and rabbits have not produced any evidence of any teratogenic effects. Use only according to the benefit/risk assessment by the responsible veterinarian. Simparica has been administered orally to 8 week old Beagle puppies at doses of 0, 1, 3, and 5 times the maximum dose of 4 mg/kg at 28 day intervals for 10 doses. No adverse effects were observed at the maximum dose of 4 mg/kg. In the overdose groups, transient and self-limiting neurological signs were observed in some animals: mild tremors at 3 times the maximum dose and convulsions at 5 times the maximum dose. All dogs recovered without treatment. Sarolaner is well tolerated in Collies with a deficient multidrug-resistance-protein 1 (MDR1 -/-) following single oral administration at 5 times the recommended dose. No treatment-related clinical signs were observed. User warnings Wash hands after handling the product. The accidental ingestion of the product may potentially result in adverse effects, such as transient excitatory neurological signs. To prevent children from accessing the product, only one chewable tablet at a time should be removed from the blister pack and only when required. The blister pack should then be returned into the carton immediately after use and the carton should be stored out of the sight and reach of children. In case of accidental ingestion, seek medical advice immediately and show the package leaflet or label to the physician. Pharmaceutical precautions Simparica does not require any special storage conditions. Any unused veterinary medicinal product or waste materials derived from such veterinary medicinal products should be disposed of in accordance with local requirements. Keep out of the sight and reach of children. For animal treatment only. Legal category Legal category: POM-V Packaging quantities Supplied in cartons containing 1, 3 or 6 tablets. Not all pack sizes may be marketed.