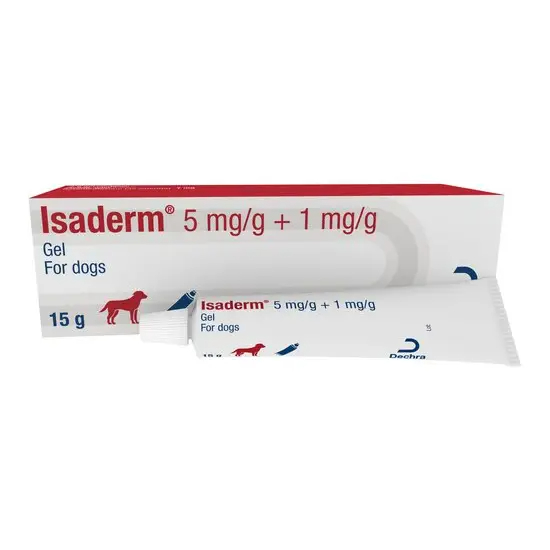
Isaderm® 5 mg/g + 1 mg/g Gel for dogs Species: Dogs Therapeutic indication: Pharmaceuticals: Antimicrobials: Topical preparations: Skin Active ingredient: Betamethasone Valerate, Fusidic Acid Product:Isaderm® 5 mg/g + 1 mg/g Gel for dogs Product index: Isaderm 5 mg/g + 1 mg/g Gel for dogs Qualitative and quantitative composition 1 g gel contains: Active substances: Fusidic acid 5 mg Betamethasone (as valerate) 1 mg Excipients: Methylparahydroxybenzoate (E218) 2.7 mg Propylparahydroxybenzoate 0.3 mg Pharmaceutical form Gel. White translucent gel. Clinical particulars Target species Dogs Indications for use For the topical treatment of surface pyoderma in the dog such as acute moist dermatitis (‘hot spots’) and intertrigo (skin fold dermatitis). Contraindications Do not use for the treatment of deep pyoderma. Do not use in pyotraumatic furunculosis and pyotraumatic folliculitis with ‘satellite’ lesions of papules or pustules. Do not use where fungal or viral infection is present. Do not apply to the eye. Do not use over large surface areas or for prolonged treatment. Do not use in cases of known hypersensitivity to the active substances or to any of the excipients. See Use during pregnancy and lactation. Special precautions for use in animals Official, national and regional antimicrobial policies should be taken into account when the product is used. Betamethasone valerate can be absorbed percutaneously and may cause temporary suppression of adrenal function. The dog should be prevented from licking treated lesions and so ingesting the product. Where there is a risk of self-trauma or a risk of accidental transfer to the eye, for example, application of the product on the forelimb, preventative measures such as the use of an Elizabethan collar should be considered. Pyoderma is often secondary in nature. The underlying cause should be identified and treated. It is recommended that use of the product should be based on bacteriological sampling and susceptibility testing. If this is not possible, therapy should be based on epidemiological information about susceptibility of the target bacteria. Use of the product deviating from the instructions given in this datasheet may increase the prevalence of bacteria resistant to fusidic acid. The safety of the combination has not been assessed in puppies of less than 7 months. Special precautions to be taken by the person administering the veterinary medicinal product to animals Corticosteroids may produce irreversible effects in the skin; they can be absorbed and may have harmful effects, especially with frequent and extensive contact or in pregnancy. Pregnant women should take special care to avoid accidental exposure. Always wear single-use disposable gloves when applying this product to animals. Wash hands after having applied the product. Care should be taken to avoid accidental ingestion by a child. In the case of accidental ingestion, seek medical advice immediately and show the package leaflet to the physician. People with known hypersensitivity to the active ingredients or to any of the excipients should avoid contact with the veterinary medicinal product. Adverse reactions Prolonged and intensive use of topical corticosteroid preparations or treatment of a large cutaneous surface (>10%) is known to trigger local or systemic effects including suppression of adrenal function, thinning of the epidermis and delayed healing. Locally applied steroids may cause depigmentation of the skin. Discontinue use if hypersensitivity develops to the product. Use during pregnancy and lactation Laboratory studies showed that topical application of betamethasone in pregnant females may lead to malformations in neonates. The safety of the product has not been assessed during pregnancy and lactation. The use of the product during pregnancy and lactation is not recommended. Amounts to be administered and administration route Cutaneous use. First, the hairs covering the lesions should be gently clipped. The affected area should then be thoroughly cleaned with an antiseptic wash before application of the gel. The amount applied should cover the affected area in a thin layer. Apply approximately 0.5 cm length of gel per 8 cm2 of lesion, twice daily, for a minimum period of 5 days. Treatment should continue for 48 hours after the lesion has resolved. The treatment period should not exceed 7 days. If there is no response within three days, or the condition deteriorates, the diagnosis should be re-evaluated. Overdose For possible signs see Adverse reactions. Pharmacological particulars Pharmacotherapeutic group: Corticosteroids, combinations with antibiotics. ATCvet code: QD07CC01 Pharmacodynamic properties Betamethasone valerate is a potent corticosteroid that possesses anti-inflammatory and anti‑pruritic properties. Fusidic acid has a steroidal structure but does not possess any steroid-like effects. It belongs to the class of antibiotics called Fusidanes. Fusidic acid acts by prohibiting the protein synthesis of bacteria when it binds to elongation factor G (required for translocation on the bacterial ribosome after peptide bond formation during protein synthesis). Its action is largely bacteriostatic, but at high concentrations (2-32-fold higher than the MIC) the effect may be bactericidal. Fusidic acid has activity against Gram‑positive bacteria, namely Staphylococcus spp. (particularly S.pseudintermedius) including penicillinase producing species. It is also active against streptococci. Pathogenic bacteria Fusidic acid Sensitive/Resistant Fusidic acid MIC Gram-positive bacteria Staphylococcus pseudintermedius Sensitve MIC90 ~ 0.25-4 µg/ml Streptococcus spp. Sensitive MIC90 ~ 8-16 µg/ml Corynebacteria spp. Sensitive MIC90 ~ 0.04-12.5 µg/ml Gram-negative bacteria Pseudomonas spp. Resistant >128 µg/ml E.coli Resistant >128 µg/ml Data based on studies conducted mainly in Europe but also in North America between 2002 and 2011. Two major mechanisms of resistance to fusidic acid have been reported in S. aureus – the alteration of the drug target site which is due to chromosomal mutations in FusA (encoding elongation factor EF-G) or FusE encoding ribosome protein L6, and the protection of the drug target site by FusB family proteins, including fusB, fusC, and fusD. The fusB determinant originally was found on the plasmid in S. aureus but has also been found on a transposon-like element or in a staphylococcal pathogenicity. No cross-resistance between fusidic acid and other antibiotics that are in clinical use has been identified. Pharmacokinetic properties In vitro data obtained from a study on dog skin indicates that 17 % of the applied dose of betamethasone and 2.5 % of the applied dose of fusidic acid are absorbed over 48 hours after the administration of the product to the skin. Absorption after administration to inflamed skin is likely to be greater.