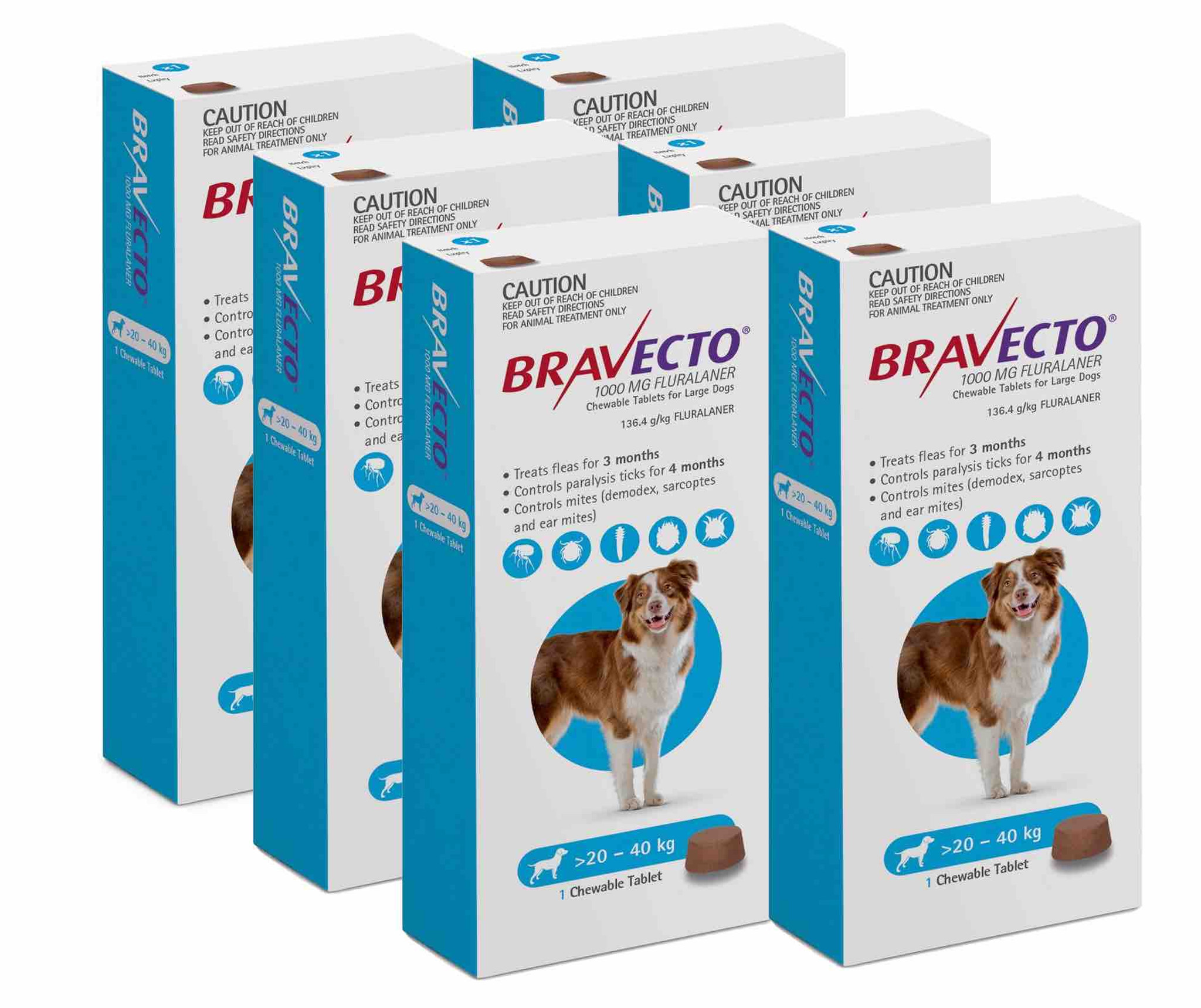
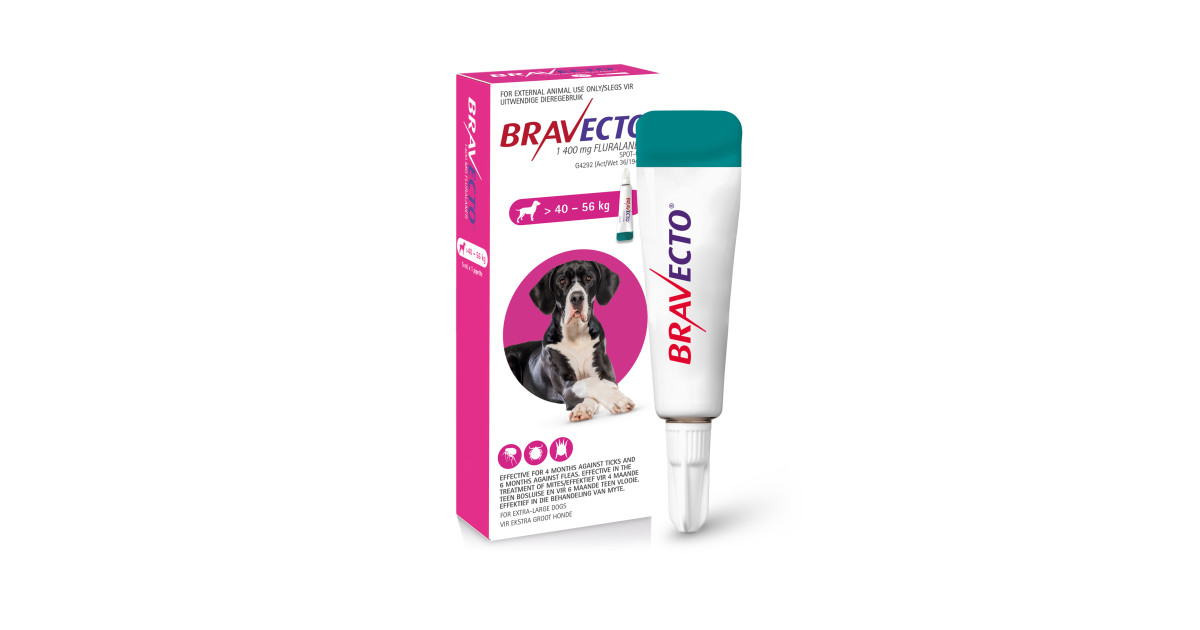
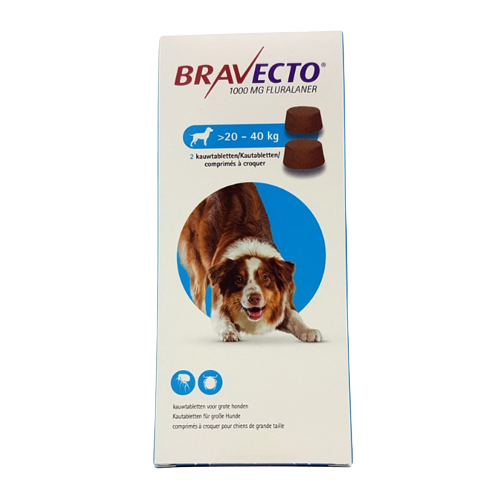
Bravecto® chewable tablets for dogs For the treatment of tick and flea infestations in dogs. Method of administration: Administer Bravecto chewable tablets at or around the time of feeding. Bravecto is a chewable tablet and is well-accepted by most dogs. If the tablet is not taken up voluntarily by the dog it can also be given with food or directly into the mouth. The dog should be observed during administration to confirm that the tablet is swallowed. The chewable tablets should not be broken or divided. For dogs above 56 kg body weight, use a combination of two tablets that most closely matches the body weight. Bravecto should be administered in accordance with the following table (corresponding to a dose of 25 – 56 mg fluralaner/kg body weight within one weight band): Body weight of dog (kg) Strength and number of tablets to be administered 112.5 mg 250 mg 500 mg 1000 mg 1400 mg 2 - 4.5 1 > 4.5 - 10 1 > 10 - 20 1 > 20 - 40 1 > 40 - 56 1 Treatment schedule For optimal control of flea infestation, the veterinary medicinal product should be administered at intervals of 12 weeks. For optimal control of tick infestation, the timing of retreatment depends on the tick species. See also section "Indications for use". Species: Dogs Therapeutic indication: Pharmaceuticals: Ectoparasiticides: For dogs Active ingredient: Fluralaner Product:Bravecto® chewable tablets for dogs Product index: Bravecto chewable tablets for dogs Qualitative and quantitative composition Each chewable tablet contains Bravecto chewable tablets Fluralaner (mg) for very small dogs 112.5 (2- 4.5 kg) for small dogs 250 (> 4.5 - 10 kg) for medium-sized dogs 500 (> 10 - 20 kg) for large dogs 1000 (> 20 - 40 kg) for very large dogs 1400 (> 40 - 56 kg) For the full list of excipients, see section "Pharmaceutical Particulars". Pharmaceutical form Chewable tablet. Light to dark brown tablet with a smooth or slightly rough surface and circular shape. Some marbling, speckles or both may be visible. Clinical particulars Target species Dogs. Indications for use For the treatment of tick and flea infestations in dogs. This veterinary medicinal product is a systemic insecticide and acaricide that provides: - immediate and persistent flea (Ctenocephalides felis) killing activity for 12 weeks, - immediate and persistent tick killing activity for 12 weeks for Ixodes ricinus, Dermacentor reticulatus and D. variabilis, - immediate and persistent tick killing activity for 8 weeks for Rhipicephalus sanguineus. Fleas and ticks must attach to the host and commence feeding in order to be exposed to the active substance. The product can be used as part of a treatment strategy for the control of flea allergy dermatitis (FAD). For the treatment of demodicosis caused by Demodex canis. For the treatment of sarcoptic mange (Sarcoptes scabiei var. canis) infestation. For reduction of the risk of infection with Babesia canis canis via transmission by Dermacentor reticulatus for up to 12 weeks. The effect is indirect due to product’s activity against the vector. Contraindications Do not use in cases of hypersensitivity to the active substance or to any of the excipients. Special warnings for each target species Parasites need to start feeding on the host to become exposed to fluralaner; therefore the risk of the transmission of parasite borne diseases (including Babesia canis canis) cannot be completely excluded. Special precautions for use Use with caution in dogs with pre-existing epilepsy. In the absence of available data, the veterinary medicinal product should not be used on puppies less than 8 weeks old and /or dogs weighing less than 2 kg. The product should not be administered at intervals shorter than 8 weeks as the safety for shorter intervals has not been tested. Operator warnings Keep the product in the original packaging until use, in order to prevent children from getting direct access to the product. Hypersensitivity reactions in humans have been reported. Do not eat, drink or smoke while handling the product. Wash hands thoroughly with soap and water immediately after use of the product. Adverse reactions Mild and transient gastrointestinal effects such as diarrhoea, vomiting, inappetence, and drooling were commonly observed in clinical trials (1.6% of treated dogs). Lethargy, muscle tremor, ataxia and convulsions have been reported very rarely in spontaneous reports. Most reported adverse reactions were self-limiting and of short duration. The frequency of adverse reactions is defined using the following convention: - very common (more than 1 in 10 animals treated displaying adverse reaction(s)) - common (more than 1 but less than 10 animals in 100 animals treated) - uncommon (more than 1 but less than 10 animals in 1,000 animals treated) - rare (more than 1 but less than 10 animals in 10,000 animals treated) - very rare (less than 1 animal in 10,000 animals treated, including isolated reports). Use during pregnancy or lactation The safety of the veterinary medicinal product in breeding, pregnant and lactating dogs has been demonstrated. Can be used in breeding, pregnant and lactating dogs. Interactions Fluralaner is highly bound to plasma proteins and might compete with other highly bound active substances such as non-steroidal anti-inflammatory drugs (NSAIDs) and the coumarin derivative warfarin. Incubation of fluralaner in the presence of carprofen or warfarin in dog plasma at maximum expected plasma concentrations did not reduce the protein binding of fluralaner, carprofen or warfarin. During clinical field testing, no interactions between Bravecto chewable tablets for dogs and routinely used veterinary medicinal products were observed. Amounts to be administered and administration route For oral use. For the treatment of Demodex canis mite infestations, a single dose of the product should be administered. As demodicosis is a multi-factorial disease, it is advisable to also treat any underlying disease appropriately. For the treatment of sarcoptic mange infestations (Sarcoptes scabiei var. canis), a single dose of the product should be administered. The need for and frequency of re-treatment should be in accordance with the advice of the prescribing veterinarian. Overdose No adverse reactions were observed following oral administration to puppies aged 8 – 9 weeks and weighing 2.0 – 3.6 kg treated with overdoses of up to 5 times the maximum recommended dose (56 mg, 168 mg and 280 mg fluralaner/kg body weight) on three occasions at shorter intervals than recommended (8-week intervals). There were no findings on reproductive performance and no findings of concern on offspring viability when fluralaner was administered orally to Beagle dogs at overdoses of up to 3 times the maximum recommended dose (up to 168 mg/kg body weight of fluralaner). The veterinary medicinal product was well tolerated in Collies with a deficient multidrug-resistance-protein 1 (MDR1 -/-) following single oral administration at 3 times the recommended dose (168 mg/kg body weight). No treatment-related clinical signs were observed. Withdrawal periods Not applicable.