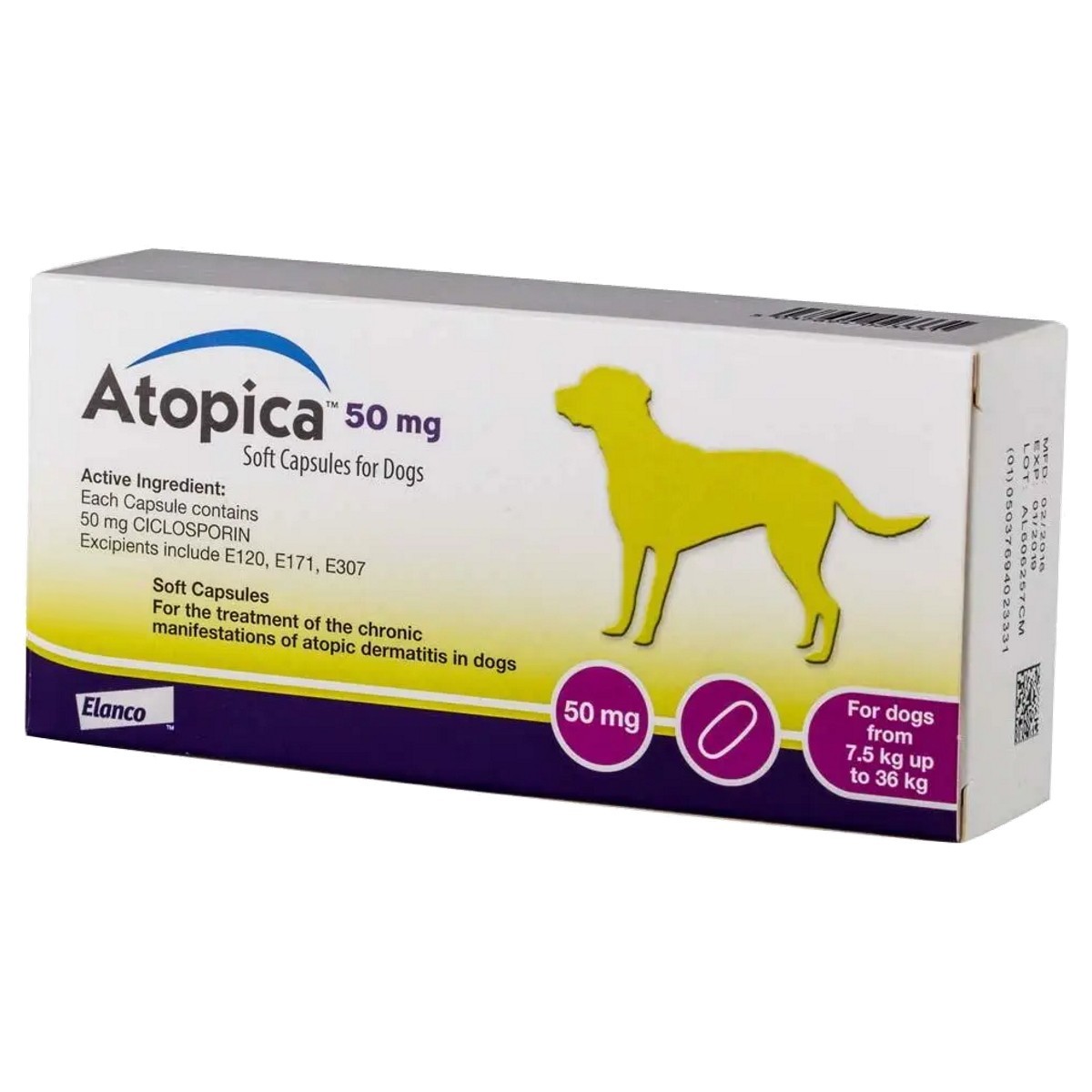
Atopica Capsules 50 mg Atopica 50mg Capsules are indicated for the treatment of chronic manifestations of atopic dermatitis in dogs weighing 7.5kg to 36kg. Atopica contains Ciclosporin as the active ingredient, which helps to reduce the body and skin’s reaction to allergens, and calms the immune system to reduce the effect of the dermatitis. Each Capsule Contains: Active Substance: Ciclosporin 50mg Excipients: α-tocopherol (E-307) 0.50mg, Titanium Dioxide (E-171) 4.50mg, Carminic Acid (E-120) <1.00μg For Dogs: Do not use Atopica in cases of hypersensitivity to ciclosporin, in dogs less than six months of age or less than 2 kg in weight or in cases with a history of malignant disorders or progressive malignant disorders. Do not vaccinate with a live vaccine whilst under treatment or within a two-week interval either before or after treatment. Clinical signs of atopic dermatitis such as pruritus and skin inflammation are not specific for this disease and therefore other causes of dermatitis such as ectoparasitic infestations, other allergies which cause dermatological signs (e.g. flea allergic dermatitis or food allergy) or bacterial and fungal infections should be ruled out before treatment is started. It is good practice to treat flea infestations before and during treatment of atopic dermatitis. It is recommended to clear bacterial and fungal infections before administering the veterinary medicinal product. However, infections occurring during treatment are not necessarily a reason for drug withdrawal, unless the infection is severe. In the presence of suggestive signs of diabetes mellitus, the effect of treatment on glycaemia must be monitored. The use of ciclosporin is not recommended in diabetic dogs or in lactating bitches is not recommended. Atopica is initially given daily until a satisfactory clinical improvement is seen which will generally be the case within 4 weeks. If no response is obtained within the first 8 weeks, the treatment should be stopped. Once the clinical signs of atopic dermatitis are satisfactorily controlled, the preparation can then be given every other day as a maintenance dose. Your vet should perform a clinical assessment at regular intervals and adjust the frequency of administration to the clinical response obtained. Adjunct treatment (e.g. medicated shampoos, fatty acids) may be considered before reducing the dosing interval. Treatment may be stopped when the clinical signs are controlled. Upon recurrence of clinical signs, treatment should be resumed at daily dosing, and in certain cases repeated treatment courses may be required. Atopica should be given at least 2 hours before or after feeding by placing the capsule directly into the dog’s mouth. The bioavailability is better and less subject to individual variations if atopica is administered to fasted animals rather than at mealtimes. Following repeated daily administration to dogs ciclosporin concentration in the skin is several times higher than in blood. Ciclosporin is metabolised mainly in the liver but also in the intestine leading to metabolites with little or no activity. Elimination is mainly via the faeces, only 10% is excreted in the urine, mostly in the form of metabolites. No significant accumulation was observed in blood of dogs treated for one year. For Cats: Allergic dermatitis in cats can have various manifestations, including eosinophillic plaque, head and neck excoriation, symmetrical alopecia and/or miliary dermatitis. Clinical signs of allergic dermatitis such as pruritus and skin inflammation are not specific for this disease and therefore other causes of dermatitis such as ectoparasitic infestations should be evaluated and eliminated. It is good practice to treat flea infestations before and during treatment of allergic dermatitis. A complete clinical examination should be performed prior to treatment. The immune status of cats to FeLV and FIV infections should be assessed before treatment. While ciclosporin does not induce tumours, it does inhibit T-lymphocytes and therefore treatment with ciclosporin may lead to an increased incidence of clinically apparent malignancy. If lymphadenopathy is observed in cats while on treatment with ciclosporin, the animal should be evaluated for clinical disease and treatment discontinued if necessary. Ciclosporin may cause elevated levels of blood glucose. The use of ciclosporin is not recommended in diabetic cats. The efficacy and safety of ciclosporin has neither been assessed in cats less than 6 months of age nor weighing less than 1.5kg. Cats that are seronegative for T. gondii may be at risk of developing clinical toxoplasmosis if they become infected while under treatment. In rare cases this can be fatal. Potential exposure of seronegative cats to Toxoplasma should therefore be avoided (eg keep indoors, avoid raw meat or scavenging). Ciclosporin was shown to not increase T. gondii oocyte shedding in a controlled laboratory study. In cases of clinical toxoplasmosis or other serious systemic illness, stop treatment with ciclosporin and initiate appropriate therapy. Any infections should be properly treated before initiation of treatment. Infections occurring during treatment are not necessarily a reason for drug withdrawal, unless the infection is severe. Treatment with Atopica oral solution may result in decreased immune response to vaccination. It is recommended not to vaccinate during treatment or within a two-week interval before or after administration of the product. Clinical studies in cats have shown that decreased appetite and weight loss may occur during ciclosporin treatment. Monitoring of body weight is recommended. In the event of persistent, progressive weight loss a complete clinical examination should be performed and treatment discontinued. It is not recommended to use immunosuppressive agents concomitantly. Special precautions to be taken by the person administering the veterinary medicinal product to animals Wash hands after administration. In case of accidental ingestion, seek medical advice immediately and show the package leaflet or the label to the physician. People with known hypersensitivity to ciclosporin should avoid contact with the product. Adverse reactions (frequency and seriousness) The most frequently observed undesirable effects are gastrointestinal disturbances such as vomiting and diarrhoea. These are generally mild and transient and do not require the cessation of the treatment. Other undesirable effects observed in clinical studies included: lethargy, anorexia, hypersalivation, weight loss and lymphopaenia. These effects generally resolve spontaneously after treatment is stopped or following a decrease in dosing frequency. Side affects may be severe in individual animals. Use during pregnancy, lactation or lay The safety of the drug has neither been studied in male reproducing cats nor in pregnant or lactating female cats. In the absence of such studies in the cat, it is only recommended to use the drug in reproducing cats only upon a positive risk/benefit assessment by the veterinary surgeon. In laboratory animals, at doses which induce maternal toxicity (rats at 30mg/kg BW and rabbits at 100mg/kg BW) ciclosporin was embryo-and foetotoxic, as indicated by increased pre- and postnatal mortality and reduced foetal weight together with skeletal retardations. In the well-tolerated dose range (rats at up to 17 mg/kg BW and rabbits at up to 30mg/kg BW) ciclosporin was without embryolethal or teratogenic effects. In laboratory animals ciclosporin passes the placenta barrier and is excreted via milk. Therefore treatment in lactating cats is not recommended. Interaction with other medicinal products and other forms of interaction Various substances are known to competitively inhibit or induce the enzymes involved in the metabolism of ciclosporin, in particular cytochrome P450 (CYP 3A 4). The compound class of azoles e.g ketoconazole is know to increase the blood concentration of ciclosporin in cats, which is considered to be clinically relevant. Macrolides such as erythromycin may increase the plasma levels of ciclosporin up to twofold. Certain inducers of cytochrome P450, anticonvulsants and antibiotics (e.g. trimethoprim/sulfadimidine) may lower the plasma concentration of ciclosporin. Ciclosporin is a substrate and an inhibitor of the MDRI P-glycoprotein transporter. Therefore, the co-administration of ciclosporin with P-glycoprotein substrates such as macrocyclic lactones could decrease the efflux of such drugs from blood-brain barrier cells, potentially resulting in signs of CNS toxicity. In clinical studies with cats treated with ciclosporin and selamectin or milbemycin, there did not appear to be an association between these drugs' concomitant use and neurotoxicity. Ciclosporin can increase the nephrotoxocity of aminoglycoside antibiotics and trimethoprim, the concomitant use of ciclosporin with these antibiotics is not recommended.